A recent headline grabbed my attention: the National Institute for Health and Care Excellence (NICE) recommends the United Kingdom’s National Health Service (NHS) cover the new weight loss medication, Wegovy. Given that England’s NHS review process was characterized as a “death panel” by some in the US not long ago, I was eager to understand the details of this recommendation. Both countries recognize obesity as a top driver of poor health and high health care spending and report that more than 60% of their populations are either overweight or obese.
By way of background, Wegovy is a once a week injectable semaglutide, a medication that mimics the GLP-1 hormone (glucagon-like peptide-1). Released after eating, GLP-1 suppresses appetite and slows gastric motility, making people feel full longer and eat less. Clinical trial data show that participants lost on average 12% more of their body weight compared to a placebo over a 68-week period. Weight loss was found to be higher when combined with a supervised weight loss program. Studies have shown that weight returns, though, when the medication is stopped, suggesting this would be a lifelong treatment. Since its June 2021 US Food and Drug Administration (FDA) approval, Novo has experienced supply chain issues which have limited the drug's availability and postponed its marketing efforts to physicians. Supply chain issues are expected to be resolved in the coming months. Early indications suggest that patient success and physician acceptance is favorable and that product awareness and use are beginning to pick up.
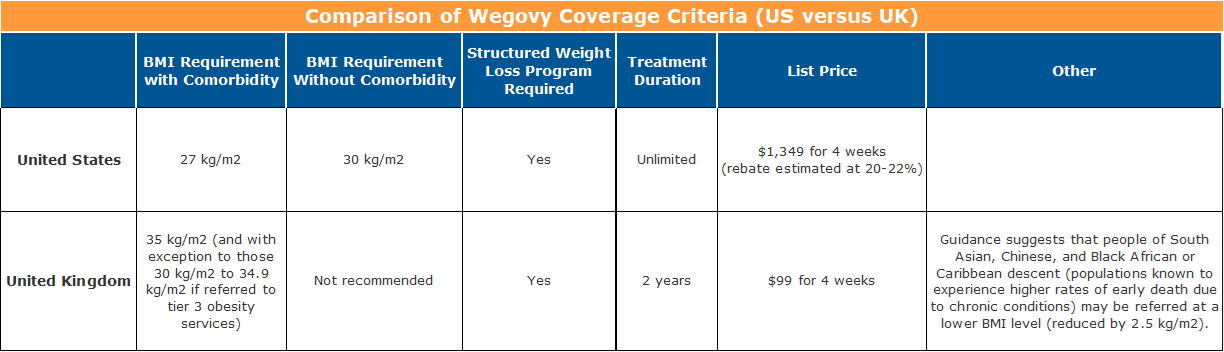
The FDA approved Wegovy for use, in addition to a reduced calorie diet and increased physical activity, for patients with at least one weight-related ailment and a body mass index (BMI) of 27 kg/m2 or greater, as well as for people with or without an obesity comorbidity at a BMI of 30 kg/m2 or greater. Weight-related conditions include high blood pressure, type 2 diabetes, or high cholesterol.
NICE’s independent appraisal committee recommended that Wegovy be considered as an option for weight management when offered alongside a supervised weight-loss program for a maximum of two years. Patients must have at least one weight-related condition and a BMI of 35 kg/m2 or greater. With exception, patients with a BMI of 30.0 kg/m2 to 34.9 kg/m2 may be considered if they meet certain additional criteria. Individuals from a South Asian, Chinese, and Black African or Caribbean background (known to experience higher rates of preventable premature death) were recommended for consideration at a lower BMI (usually reduced by 2.5 kg/m2).
But what about the price differences?
Novo has set the list price for Wegovy in the US at $1,349 for a four-week supply. Rebates are generally understood to be about 20% to 22% of the list price. This means that net of rebates, Wegovy will cost over $13,000 per patient per year in the US.
In the UK, not so much. Wegovy has a list price of about $99.08 (£73.25) for a four-week supply, or just under $1,300 per patient per year. So before any negotiations off of the list price, the annual cost in the UK is less than a one-month, after-rebate supply in the US.
We all know that the US has a very different and cumbersome supply chain, especially when compared to a single-payer system like that in the UK. So I was prepared for a price that was double, triple, or maybe quadruple – but not ten times or more.
Why is it priced so much higher in the US? Well, it seems that it is the usual answer…“because it can." Wegovy is but one example of a problem we see often in the US pharmaceutical industry.
The Institute for Clinical and Economic Review (ICER), the US' non-profit, private-sector response to the UK’s NICE, has announced that it will undertake a review of Wegovy this fall. Using an open, transparent, and independent consensus-based process, ICER reviews medications and establishes a fair price for use in the US. ICER leaders have been giving a lot of thought to how employers in the US could leverage this work to achieve better value for their investments in pharmaceuticals. Several possible solutions in their forthcoming Purchaser Playbook hold appeal. BHC members can get a sneak peek at these recommendations, and we look forward to receiving your feedback. Let’s not despair. Let’s take action.
Warm Regards,
Louise Y. Probst
BHC Executive Director